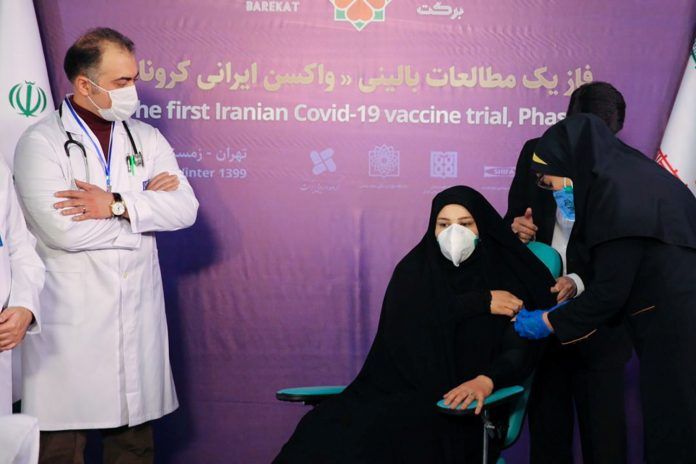
DUBAI, Dec 29 (Reuters) – Iran launched human trials of its first domestic COVID-19 vaccine candidate, state media reported on Tuesday, which Tehran says could help it defeat the pandemic despite U.S. sanctions interfering with its ability to import vaccines.
Setad, a giant state-affiliated conglomerate controlled by Iran’s supreme leader, said production of the vaccine developed by one of its companies, Shifa Pharmed, could reach 12 million doses per month, six months after a successful trial ends.
The first volunteers to take the vaccine were officials of the conglomerate and the daughter of its head, in an apparent effort to boost public confidence in the vaccine.
“The message of this act was that we do not see ourselves apart from the people, and we brought our family first to test this vaccine,” Health Minister Saeed Namaki said, according to state broadcaster IRIB.
Iran has been the worst-hit country in the Middle East by the global COVID-19 pandemic. It complains that its ability to buy vaccines is hampered by U.S. financial sanctions, reimposed after the Trump administration abandoned a 2015 nuclear agreement. Food and medicine are exempt from the sanctions, but banks have been discouraged from financing Iranian deals.
Tehran said last week it had received approval from U.S. authorities to buy coronavirus vaccines from the World Health Organization-led COVAX alliance.
Iran’s Red Crescent Society has said that, separately from the government, it was planning to import a Chinese vaccine.
(Reporting by Dubai newsroom Editing by Peter Graff)